New Delhi
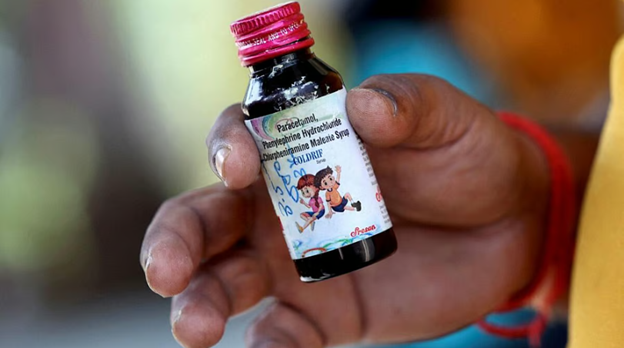
The investigation into Coldrif cough syrup, linked to the deaths of children in Madhya Pradesh, has highlighted serious lapses by the Tamil Nadu Food and Drug Administration (TNFDA) in enforcing regulatory norms, according to sources from the Central Drugs Standard Control Organisation (CDSCO).
A recent CDSCO inspection exposed the unit’s appalling conditions and total non-compliance with Good Manufacturing Practices (GMP). The company had not registered its products on the national ‘Sugam’ portal, a mandatory requirement under Rule 84AB of the Drugs and Cosmetics Rules to maintain a centralized database for better monitoring. Despite repeated communications and reminders from CDSCO, neither the company complied nor did TNFDA assist in registration.
TNFDA conducted an audit of Sresan Pharma on October 1-2 following a request from Madhya Pradesh authorities but did not share the findings with CDSCO. During a joint risk-based inspection on October 3, TNFDA officials failed to participate, leaving CDSCO to carry out the audit independently and recommend license cancellation. TNFDA also withheld information about Coldrif syrup analysis, which later revealed 48% DEG content against the permissible 0.1%, creating confusion among regulatory bodies.
The CDSCO has advised TNFDA to cancel Sresan Pharma’s license and consider criminal action. Meanwhile, the MP Police arrested the company’s owner on October 8. Sresan Pharma is neither WHO GMP certified nor compliant with Revised Schedule M requirements. A high-level meeting chaired by Union Health Secretary Punya Salila Srivastava emphasized strict action and better enforcement of drug quality norms, particularly for paediatric medications.